Tritium Mass
Tritium is an isotope of hydrogen with on proton and two neutrons.
The Math / Science
Tritium is a common element in the development of fusion nuclear energy. Tritium is less common than other hydrogen isotopes (protium and deuterium) in Earth's environment. However, it is created in the upper atmosphere from cosmic rays. The manufacturing of tritium is possible by bombarding lithium with neutrons. While radioactive, tritium has a short half-life of a little over twelve (12) years. This means that it naturally decays into a non-radioactive element, Helium 3, rapidly and poses no long-term radioactive or storage issues.
As a plasma, Tritium is a common ingredient in fusion energy. The equation for Nuclear Binding Energy calculates the energy released in the formation of an atom from subatomic particles. This can be expressed in a rewritten form of the Einstein relationship:
E = Δm⋅c2
Where:
- E = nuclear binding energy
- Δm = difference in mass of the alpha particle and the sum of its parts (protons and neutrons)
- c2 = speed of light squared (931.46 MeV/u)
Nuclear binding energy is the energy equivalent of the mass deficiency of an atom. Mass deficiency is the amount of matter that would be converted into energy if an atom were constructed from fundamental particles. So, essentially, nuclear binding energy is the energy released from an atom if it were formed from individual protons and neutrons.
Nuclear Fusion
In the most common fusion experiments, Deuterium and Tritium 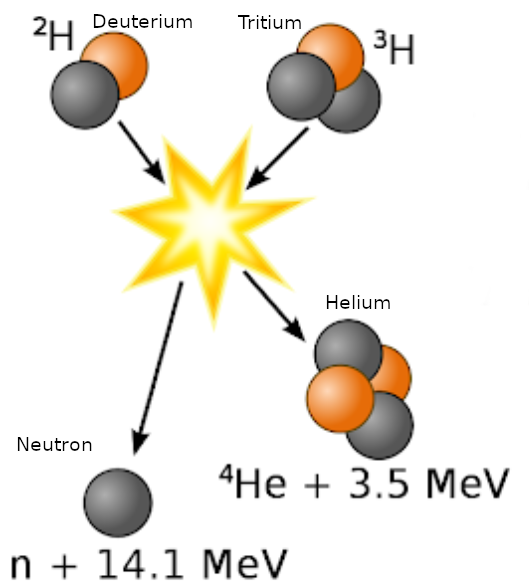 are super heated in devices like a tokamak at ITER to cause a fusion reaction (see graphic). This is known as a DT reaction. In a DT reaction, the Deuterium and Tritium are fused together and create a Helium 4 atom with an energy state of 3.5 MeV, and a highly energetic neutron at 14.1 MeV. The following lists their mass in atomic mass units (u):
are super heated in devices like a tokamak at ITER to cause a fusion reaction (see graphic). This is known as a DT reaction. In a DT reaction, the Deuterium and Tritium are fused together and create a Helium 4 atom with an energy state of 3.5 MeV, and a highly energetic neutron at 14.1 MeV. The following lists their mass in atomic mass units (u):
- Deuterium (2H): 2.01410177811 u
- Tritium (3H) : 3.016049281 u
to
- Helium (4He): 4.00260325413 u
- Neutron: 1.0086649158 u
The delta in mass is `Delta_m `= 0.0188828 u. If you use the binding energy formula in this calculator, the released energy equivalent to 0.0188828 u is equal to 17.588 MeV, which is the sum of the 14.1 MeV neutron and the 3.5 MeV Helium atom in the graphic above.
- Comments
- Attachments
- Stats
No comments |
