The Kinetic Energy calculator computes the kinetic energy (KE) of an object based on its mass (m) and velocity (v).
INSTRUCTIONS: Choose units and enter the following:
- (m) - the mass of the object
- (v) - the velocity of the object
Kinetic Energy: The calculator returns the energy (KE) in joules. However this can be automatically converted to other energy units via the pull-down menu.
The Math / Science
The formula for Kinetic Energy is as follows:
where:
- KE is the kinetic energy
- m is the mass of the object
- v is the velocity
Kinetic Energy is the energy that exists in a moving body at a specified velocity or the energy required to accelerate a body to a specified velocity.
Energy Calculators
- Kinetic Energy: `KE= 1/2 *m * v^2`
- Kinetic Energy (change of velocity): `KE = 1/2*m*(V_1-V_2)^2`
- Relativistic Kinetic Energy: `E_K = (m*c^2)/sqrt(1 - v^2"/"c^2") - m*c^2`
- Potential Energy: `U(y) = m*g*y`
- Potential Energy of Gravity (two bodies): `U(G) = - (G*m1*m2)/r`
- Nuclear Binding Energy: `E = m*c^2`
- Quantum Energy (Planck's Equation): `E = h*f`
- Energy of a Particle in a Box: `E_n=(n^2h^2)/(8mL^2)`
- Molecular Kinetic Energy: `KE = 3/2 * k_B *T`
- Electrostatic Potential Energy: `E_(el) = k_e * (Q_1*Q_2)/d`
- Photon Energy from Wavelength: `E = (h*c)/lambda`
- Heat Energy to Change Material Temperature: `Q = C * m *DeltaT`
DERIVATION
An object can be fundamentally modeled as a mass concentrated at point small, a point small enough to be considered a"point mass'. In classical mechanics, the kinetic energy of a point mass (or a non-rotating rigid body) is a function of the mass of the body and its velocity.
Applying a force to a mass by definition will accelerate the mass. And we apply the following kinematic equation for final velocity to find out what the final velocity is when we have accelerated at a constant acceleration for some distance, d: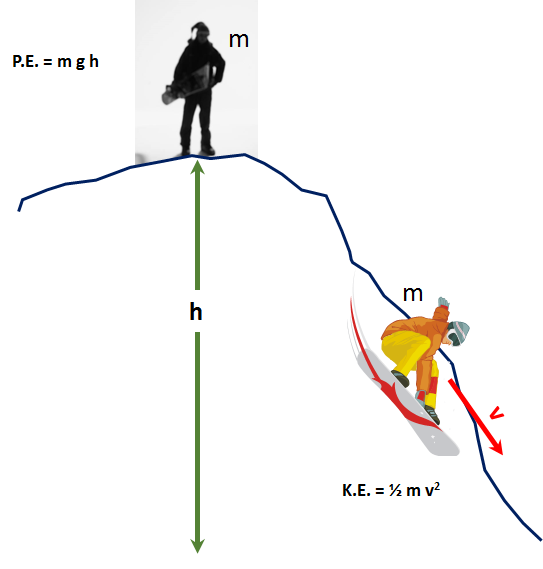
[1] `v_f^2 = v_i^2 + 2 *a *d`
Since in this kinetic energy case we are starting with no velocity and accelerating to a final velocity, `V_f`, we have:
[2] `v_f^2 = 2 *a *d`
Using [3] F = m•a, we rearrange to get: [4] `a = F/m`
Then substituting [4] into [2] we get:
[5] `v_f^2 = 2 * (F *d)/m`
We then realize that [6] F•d = "Work" and substitute Work (W) into [5], and re-arrange to solve for W.
[7] `W = (m * V_f^2) / 2`
This W is the work done to accelerate the mass to the final velocity, `V_f` and, by definition this is the Kinetic Energy.
So, finally: `K.E. = 1/2 * m*V^2`
This is one of numerous formulas for finding the kinetic energy (`KE`) of some mass, m.
You can enter any combination of mass and velocity units into this equation but if you enter kilograms for mass and m/sec for velocity, the result will be in Joules = `(kg * m) / sec^2`.
Kinetic Energy in Chemistry
written by Hunter Metcalfe
Kinetic Energy is the energy an object has because the object is in motion. To put an object, a mass, in motion you have to impart energy to the object, which is a reflection of Newton's First Law of Motion
| Viewed in an inertial reference frame, an object either remains at rest or continues to move at a constant velocity, unless acted upon by an external force. |
Increasing an object's kinetic energy causes an object at rest to no longer be at rest and increasing kinetic energy of an object that is moving at a constant velocity will increase the object's velocity. So, you must act on an object with an external force to give it velocity and thus give it kinetic energy. Note that if the velocity is zero, the kinetic energy by this definition becomes zero. That does not mean that if you stop something there is no energy in the system. Stopping a moving car does not generate a burst of energy but typically the kinetic energy is converted to some form of potential energy. In the case of a car the kinetic energy is converted to heat energy in the brakes. Converting kinetic energy to frictional heat energy may impart energy to molecules in gases or liquids whose molecules then move faster, meaning their kinetic energy has increased.
So, kinetic energy and potential energy are always trading back and forth, which is very relevant to the chemistry domain.
Kinetic energy in a car accident might be converted to energy causing deformation of the car and the object it strikes, as when a car moving at a constant velocity hits a brick wall. The kinetic energy is transmitted into the frame of the car and the structure of the wall creates deforming damage and heat.
In the case of kinetic energy as applied to chemistry, the examples are easier to understand when you take a chemical potential energy, let's say that of an explosive or highly reactive chemical element and you cause that chemical potential energy to be released. Picture the potential energy of automotive gasoline combusting and driving a car's piston. That is the process of converting the chemical potential energy of the bonds between the atoms in the gasoline chemical composition into the kinetic energy of the piston and transmitted that kinetic energy in turn to the car's wheels causing the car to have velocity and thus kinetic energy.
Pressure of a contained gas is also an atomic or molecular level example of kinetic energy. The gas molecules move at velocities that increase with increased temperature. Remember PV = nRT? This is because the moving molecules strike the walls of their container imparting their kinetic energy in to the force that causes the outward pressure. Increasing temperature of the molecules causes their travel velocity to increase, which increases their kinetic energy, which in turn increases the pressure on the walls of the container.
