0.2 What is physics by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.
0.2 What is physics?
Given for one instant an intelligence which could comprehend all the forces by which nature is animated and the respective positions of the things which compose it...nothing would be uncertain, and the future as the past would be laid out before its eyes. -- Pierre Simon de Laplace
Physics is the use of the scientific method to find out the basic principles governing light and matter, and to discover the implications of those laws. Part of what distinguishes the modern outlook from the ancient mind-set is the assumption that there are rules by which the universe functions, and that those laws can be at least partially understood by humans. From the Age of Reason through the nineteenth century, many scientists began to be convinced that the laws of nature not only could be known but, as claimed by Laplace, those laws could in principle be used to predict everything about the universe's future if complete information was available about the present state of all light and matter. In subsequent sections, I'll describe two general types of limitations on prediction using the laws of physics, which were only recognized in the twentieth century.
Matter can be defined as anything that is affected by gravity, i.e., that has weight or would have weight if it was near the Earth or another star or planet massive enough to produce measurable gravity. Light can be defined as anything that can travel from one place to another through empty space and can influence matter, but has no weight. For example, sunlight can influence your body by heating it or by damaging your DNA and giving you skin cancer. The physicist's definition of light includes a variety of phenomena that are not visible to the eye, including radio waves, microwaves, x-rays, and gamma rays. These are the “colors” of light that do not happen to fall within the narrow violet-to-red range of the rainbow that we can see.
self-check: At the turn of the 20th century, a strange new phenomenon was discovered in vacuum tubes: mysterious rays of unknown origin and nature. These rays are the same as the ones that shoot from the back of your TV's picture tube and hit the front to make the picture. Physicists in 1895 didn't have the faintest idea what the rays were, so they simply named them “cathode rays,” after the name for the electrical contact from which they sprang. A fierce debate raged, complete with nationalistic overtones, over whether the rays were a form of light or of matter. What would they have had to do in order to settle the issue?
(answer in the back of the PDF version of the book)
 Many physical phenomena are not themselves light or matter, but are properties of light or matter or interactions between light and matter. For instance, motion is a property of all light and some matter, but it is not itself light or matter. The pressure that keeps a bicycle tire blown up is an interaction between the air and the tire. Pressure is not a form of matter in and of itself. It is as much a property of the tire as of the air. Analogously, sisterhood and employment are relationships among people but are not people themselves.
Many physical phenomena are not themselves light or matter, but are properties of light or matter or interactions between light and matter. For instance, motion is a property of all light and some matter, but it is not itself light or matter. The pressure that keeps a bicycle tire blown up is an interaction between the air and the tire. Pressure is not a form of matter in and of itself. It is as much a property of the tire as of the air. Analogously, sisterhood and employment are relationships among people but are not people themselves.
Some things that appear weightless actually do have weight, and so qualify as matter. Air has weight, and is thus a form of matter even though a cubic inch of air weighs less than a grain of sand. A helium balloon has weight, but is kept from falling by the force of the surrounding more dense air, which pushes up on it. Astronauts in orbit around the Earth have weight, and are falling along a curved arc, but they are moving so fast that the curved arc of their fall is broad enough to carry them all the way around the Earth in a circle. They perceive themselves as being weightless because their space capsule is falling along with them, and the floor therefore does not push up on their feet.
Optional Topic: Modern Changes in the Definition of Light and Matter
Einstein predicted as a consequence of his theory of relativity that light would after all be affected by gravity, although the effect would be extremely weak under normal conditions. His prediction was borne out by observations of the bending of light rays from stars as they passed close to the sun on their way to the Earth. Einstein's theory also implied the existence of black holes, stars so massive and compact that their intense gravity would not even allow light to escape. (These days there is strong evidence that black holes exist.)
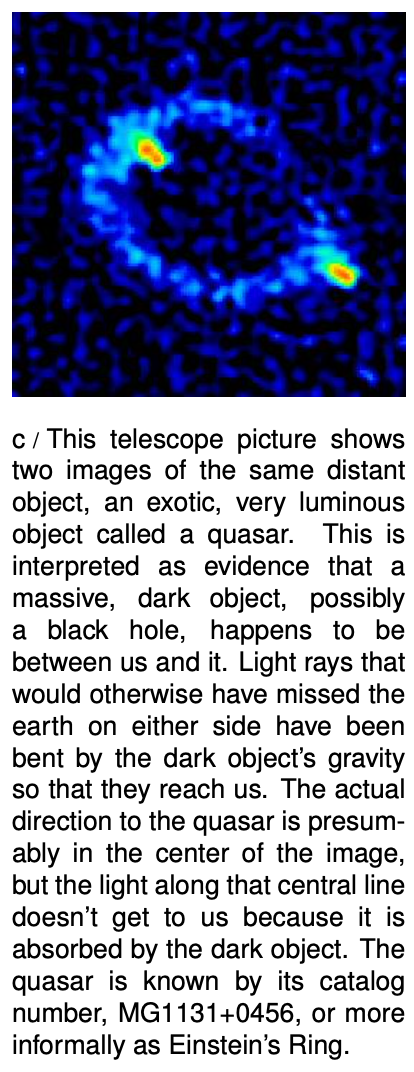
 Einstein's interpretation was that light doesn't really have mass, but that energy is affected by gravity just like mass is. The energy in a light beam is equivalent to a certain amount of mass, given by the famous equation E=mc2, where c is the speed of light. Because the speed of light is such a big number, a large amount of energy is equivalent to only a very small amount of mass, so the gravitational force on a light ray can be ignored for most practical purposes.
Einstein's interpretation was that light doesn't really have mass, but that energy is affected by gravity just like mass is. The energy in a light beam is equivalent to a certain amount of mass, given by the famous equation E=mc2, where c is the speed of light. Because the speed of light is such a big number, a large amount of energy is equivalent to only a very small amount of mass, so the gravitational force on a light ray can be ignored for most practical purposes.
There is however a more satisfactory and fundamental distinction between light and matter, which should be understandable to you if you have had a chemistry course. In chemistry, one learns that electrons obey the Pauli exclusion principle, which forbids more than one electron from occupying the same orbital if they have the same spin. The Pauli exclusion principle is obeyed by the subatomic particles of which matter is composed, but disobeyed by the particles, called photons, of which a beam of light is made.
Einstein's theory of relativity is discussed more fully in book 6 of this series.
The boundary between physics and the other sciences is not always clear. For instance, chemists study atoms and molecules, which are what matter is built from, and there are some scientists who would be equally willing to call themselves physical chemists or chemical physicists. It might seem that the distinction between physics and biology would be clearer, since physics seems to deal with inanimate objects. In fact, almost all physicists would agree that the basic laws of physics that apply to molecules in a test tube work equally well for the combination of molecules that constitutes a bacterium. (Some might believe that something more happens in the minds of humans, or even those of cats and dogs.) What differentiates physics from biology is that many of the scientific theories that describe living things, while ultimately resulting from the fundamental laws of physics, cannot be rigorously derived from physical principles.
Isolated systems and reductionism
To avoid having to study everything at once, scientists isolate the things they are trying to study. For instance, a physicist who wants to study the motion of a rotating gyroscope would probably prefer that it be isolated from vibrations and air currents. Even in biology, where field work is indispensable for understanding how living things relate to their entire environment, it is interesting to note the vital historical role played by Darwin's study of the Galápagos Islands, which were conveniently isolated from the rest of the world. Any part of the universe that is considered apart from the rest can be called a “system.”
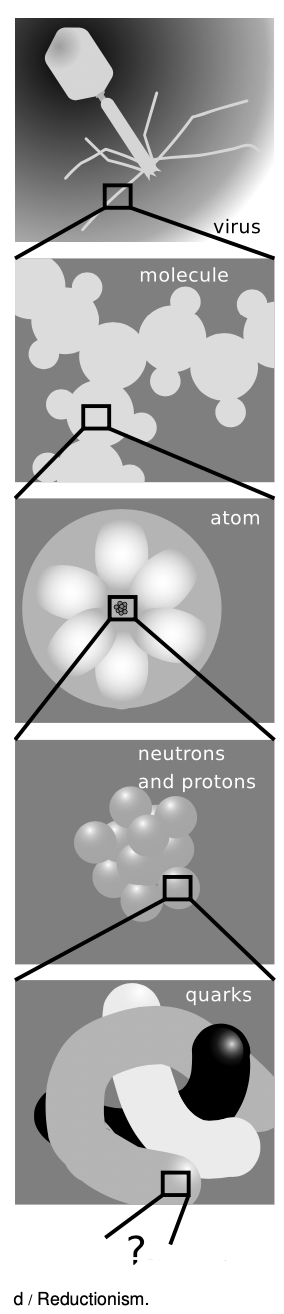
Physics has had some of its greatest successes by carrying this process of isolation to extremes, subdividing the universe into smaller and smaller parts. Matter can be divided into atoms, and the behavior of individual atoms can be studied. Atoms can be split apart into their constituent neutrons, protons and electrons. Protons and neutrons appear to be made out of even smaller particles called quarks, and there have even been some claims of experimental evidence that quarks have smaller parts inside them. This method of splitting things into smaller and smaller parts and studying how those parts influence each other is called reductionism. The hope is that the seemingly complex rules governing the larger units can be better understood in terms of simpler rules governing the smaller units. To appreciate what reductionism has done for science, it is only necessary to examine a 19th-century chemistry textbook. At that time, the existence of atoms was still doubted by some, electrons were not even suspected to exist, and almost nothing was understood of what basic rules governed the way atoms interacted with each other in chemical reactions. Students had to memorize long lists of chemicals and their reactions, and there was no way to understand any of it systematically. Today, the student only needs to remember a small set of rules about how atoms interact, for instance that atoms of one element cannot be converted into another via chemical reactions, or that atoms from the right side of the periodic table tend to form strong bonds with atoms from the left side.
Discussion Questions
A I've suggested replacing the ordinary dictionary definition of light with a more technical, more precise one that involves weightlessness. It's still possible, though, that the stuff a lightbulb makes, ordinarily called “light,” does have some small amount of weight. Suggest an experiment to attempt to measure whether it does.
B Heat is weightless (i.e., an object becomes no heavier when heated), and can travel across an empty room from the fireplace to your skin, where it influences you by heating you. Should heat therefore be considered a form of light by our definition? Why or why not?
C Similarly, should sound be considered a form of light?
0.2 What is physics by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.