Gay-Lussac's Law (of temperature/pressure) Calculator
The Gay-Lussac's Law calculator computes the initial and final pressure and temperature of an ideal gas based on Gay-Lussac's formula (T1•P2=T2•P1). The calculator automatically handles numerous temperature and pressure units. All of these calculations presume the volume and mass of the ideal gas remains static throughout. Gay-Lussac formula is:
T1•P2=T2•P1
where:
- (T1) Initial Temperature
- (P2) Final Pressure
- (T2) Final Temperature
- (P1) Initial Pressure
 The Math / Science
The Math / Science
This calculator accesses the following equations from its four buttons:
Gay-Lussac Law (final temperature) - equation computing final temperature of an ideal gas given knowledge of the initial temperature/pressure state of the gas before a pressure change
Gay-Lussac Law (initial temperature) - equation computing initial temperature of an ideal gas given knowledge of the final temperature/pressure state of the gas after a pressure change
Gay-Lussac Law (final pressure) - equation computing resultant pressure of an ideal gas given knowledge of the initial temperature/pressure state of the gas before a temperature change
Gay-Lussac Law (initial pressure) - equation computing initial pressure of an ideal gas given knowledge of the final temperature/pressure state of the gas after a temperature change
Chemistry Calculators
- R - Gas Constant: 8.3144626181532 J/(K⋅mol)
- Boyle's Law Calculator: P1 • V1 = P2 • V2
- Charles Law Calculator: V1• T2 = V2 • T1
- Combined Gas Law Calculator: P•V / T= k
- Gay-Lussac Law: T1•P2 =T2•P1
- Ideal Gas Law: P•V = n•R•T
- Bragg's Law: n·λ = 2d·sinθ
- Hess' Law: ΔH0rxn=ΔH0a+ΔH0b+ΔH0c+ΔH0d
- Internal Energy: ΔU = q + ω
- Activation Energy: Ea = (R*T1⋅T2)/(T1 - T2) ⋅ ln(k1/k2)
- Arrhenius Equation: k = AeE_a/(RT)
- Clausius-Clapeyron Equation: ln(P2/P1) = (ΔHvap)/R * (1/T1 - 1/T2)
- Compressibility Factor: Z = (p*Vm)/(R*T)
- Peng-Robinson Equation of State: p = (R*T)/(Vm - b) - (a*α)/(Vm2 + 2*b*Vm - b2)
- Reduced Specific Volume: vr = v/(R* Tcr / Pc)
- Van't Hoff Equation: ΔH0 = R * ( -ln(K2/K1))/ (1/T1 - 1/T2)
REFERENCE
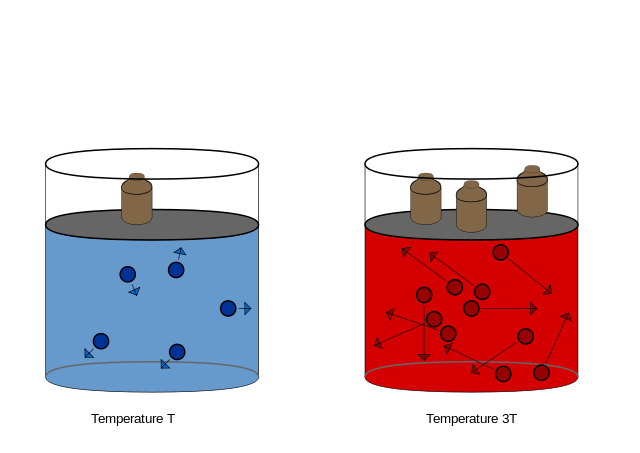
[1] Temperature Pressure law
Source: Wikipedia / Evan Mason
URL: https://en.wikipedia.org/wiki/Gay-Lussac%27s_law#mediaviewer/File:Temperature_Pressure_law.svg
Public License: CC Attribution-ShareAlike 3.0 Unported
Equations and Data Items
Collections
- Comments
- Attachments
- Stats
No comments |

