Hydroxide Ion concentration
Tags | |
UUID | 96d941ef-1864-11e6-9770-bc764e2038f2 |
Hydroxide ion[1][4] (OH-) can be obtained from a known concentration of a hydronium concentration and the equation Kw=[H+][OH-]. This equation is known as equilibrium of water and originates from the acid-base reaction of water, also known as autoionization of water. Which occurs by the following process, as illustrated:
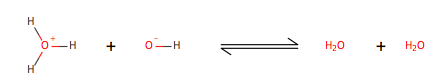
Two water molecules collide they exchange a proton, a positively charged particle, in this case it is a hydrogen missing an electron. One of the water molecules acts as an acid and the other acts as a base. There are three different acid-base theories. Bronsted-Lowery theory suggests that an acid donates a proton to the base, Lewis theory suggests that an acid accepts electrons from the base, and Arrhenius theory suggests that an acid is any molecule that increases the hydronium concentration in water and a base increases the concentration of a hydroxide. Since water autoionizes Arrhenius theory does not explain the formation of either the hydroxide or hydronium ions, which are known to be as conjugate base (OH-) and conjugate acid(H+). At 25oC the concentration of each ions is 1.0x10-7 (mol/L), when both of the concentrations remain equal then the solution becomes neutral.
Description
The equation is
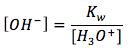 [2]
[2]
where
[H3O+] is the hydronium ion concentration in units of (mol/L)
- Kw is the ionization constant of water, which equals to
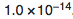 . [3]
. [3] - [OH-] is the hydroxide ion concentration in units of (mol/L)
Related Information
- Hydronium ion concentration (for the Spanisg site click here)
- pH/pOH calculator (for the Spanish site click here)
Supplement Material
- Khan Academy:Autoionization of water
References
[1]https://en.wikipedia.org/wiki/Hydroxide
[2]Whitten, et al. 10th Edition. Pp.713
[3]https://en.wikipedia.org/wiki/Self-ionization_of_water
[4] For the Spanish site click here
Collections
- Comments
- Attachments
- Stats
No comments |
