Tags | |
UUID | da77c8b3-1de2-11e6-9770-bc764e2038f2 |
The Henry's Law equation calculates the relationship between the pressure[4] and solubility of gas[5] in liquid[6]. Discovered by an English Chemist William Henry (1774-1836)[3]. The concentration of the dissolved gas in solvent[7] is usually calculated for a gas dissolved in water at 20oC (293.15K). This equation came from an observation that the solubility of gases will increase if the partial pressure of the gas molecules above the solution also increases. In short, the law states that at equilibrium the amount of gas molecules dissolved in a volume of liquid is directly proportional to the partial pressure that is in contact with the liquid.
Description
Henry's Law is calculated by:
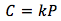 [2]
[2]
Where :
- C is the concentration of gas dissolved in the liquid in units of (mol/L)
- k is Henry's Law constant in units of (mol/L * atm)
- P is the partial pressure of gas in units of (atm)
An extensive list of Henry's Law constants can be found here. [8]
*Note: gases that are soluble in water do not obey Henry's Law.
Related Topics
Supplement Material
- Khan Academy: Henry's Law
- Crash Course: Partial Pressures & Vapor Pressure
- Henry's Law: Relationship Between Pressure and Solubility of a Gas
References
[1]Henry's law. (n.d.).
Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/Henry's_law
[2] Whitten, et al. 10th Edition. Pp.514
[3]William Henry (chemist). (n.d.).
Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/William_Henry_(chemist)
[4]Pressure. (n.d.). Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/Pressure
[5]Gas. (n.d.). Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/Gas
[6]Liquid. (n.d.). Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/Liquid
[7]Solvent. (n.d.). Retrieved May 26, 2016, from https://en.wikipedia.org/wiki/Solvent
Collections
- Comments
- Attachments
- Stats
No comments |
