Tags | |
UUID | 19d32f9b-f145-11e9-8682-bc764e2038f2 |
Amphoterism
From UCDavis Chemwiki
Molecules or ions which can either donate or accept a proton, depending on their circumstances, are called amphiprotic or amphoteric species. The most important amphiprotic species is water itself. When an acid donates a proton to water, the water molecule is a proton acceptor, and hence a base. Conversely, when a base reacts with water, a water molecule donates a proton, and hence acts as an acid.
Another important group of amphiprotic species is the amino acids. Each amino acid molecule contains an acidic carboxyl group and a basic amino group. In fact the amino acids usually exist in zwitterion (German for “double ion”) form, where the proton has transferred from the carboxyl to the amino group. In the case of glycine, for example, the zwitterion is
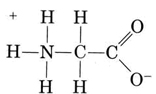
The zwitterion can donate one of the protons from the N, just as an NH4+ ion can donate a proton. On the other hand, its COO– end can accept a proton, just as a CH3COO– ion can.
Other common amphiprotic species are H2O, HCO3–, H2PO4–, HPO42–, and other anions derived from diprotic or triprotic acids.
Example 6
Write equations to show the amphiprotic behavior of
(a)
(b)
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
