LM 36.6 Atoms with more than one electron Collection
Tags | |
UUID | 1f25e965-f145-11e9-8682-bc764e2038f2 |
36.6 Atoms with more than one electron by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.
36.6 Atoms with more than one electron
What about other atoms besides hydrogen? It would seem that things would get much more complex with the addition of a second electron. A hydrogen atom only has one particle that moves around much, since the nucleus is so heavy and nearly immobile. Helium, with two, would be a mess. Instead of a wave function whose square tells us the probability of finding a single electron at any given location in space, a helium atom would need to have a wave function whose square would tell us the probability of finding two electrons at any given combination of points. Ouch! In addition, we would have the extra complication of the electrical interaction between the two electrons, rather than being able to imagine everything in terms of an electron moving in a static field of force created by the nucleus alone.
Despite all this, it turns out that we can get a surprisingly good description of many-electron atoms simply by assuming the electrons can occupy the same standing-wave patterns that exist in a hydrogen atom. The ground state of helium, for example, would have both electrons in states that are very similar to the n=1 states of hydrogen. The second-lowest-energy state of helium would have one electron in an n=1 state, and the other in n=2 states. The relatively complex spectra of elements heavier than hydrogen can be understood as arising from the great number of possible combinations of states for the electrons.
A surprising thing happens, however, with lithium, the three-electron atom. We would expect the ground state of this atom to be one in which all three electrons settle down into n=1 states. What really happens is that two electrons go into n=1 states, but the third stays up in an n=2 state. This is a consequence of a new principle of physics:
There are two n=1 states, one with sz=+1/2 and one with sz=-1/2, but there is no third n=1 state for lithium's third electron to occupy, so it is forced to go into an n=2 state.
It can be proved mathematically that the Pauli exclusion principle applies to any type of particle that has half-integer spin. Thus two neutrons can never occupy the same state, and likewise for two protons. Photons, however, are immune to the exclusion principle because their spin is an integer. Material objects can't pass through each other, but beams of light can. With a little oversimplification, we can say that the basic reason is that the exclusion principle applies to one but not to the other.1
Photons are made out electric and magnetic fields, which are directly measurable, but the wave function of a spin-1/2 particle is not observable (p. 963). The exclusion principle offers a more fundamental explanation of this difference between light and matter. We saw in example 2 on p. 947 that in a typical light wave, a huge number of photons overlap one another within a volume of one cubic wavelength, and this is what allows us to measure the amplitude and phase of the wave with a device like an antenna. But for electrons, the exclusion principle prevents us from having more than one particle in such a volume, so we can't perform this type of classical measurement of the wave.
Deriving the periodic table
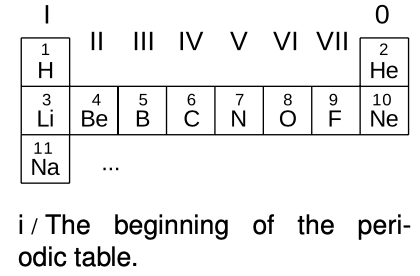 We can now account for the structure of the periodic table, which seemed so mysterious even to its inventor Mendeleev. The first row consists of atoms with electrons only in the n=1 states:
We can now account for the structure of the periodic table, which seemed so mysterious even to its inventor Mendeleev. The first row consists of atoms with electrons only in the n=1 states:
| H | 1 electron in an n=1 state |
| He | 2 electrons in the two n=1 states |
The next row is built by filling the n=2 energy levels:
| Li | 2 electrons in n=1 states, 1 electron in an n=2 state |
| Be | 2 electrons in n=1 states, 2 electrons in n=2 states |
| … | |
| O | 2 electrons in n=1 states, 6 electrons in n=2 states |
| F | 2 electrons in n=1 states, 7 electrons in n=2 states |
| Ne | 2 electrons in n=1 states, 8 electrons in n=2 states |
In the third row we start in on the n=3 levels:
| Na | 2 electrons in n=1 states, 8 electrons in n=2 states, 1 electron in an n=3 state |
| ... |
We can now see a logical link between the filling of the energy levels and the structure of the periodic table. Column 0, for example, consists of atoms with the right number of electrons to fill all the available states up to a certain value of n. Column I contains atoms like lithium that have just one electron more than that.
 This shows that the columns relate to the filling of energy levels, but why does that have anything to do with chemistry? Why, for example, are the elements in columns I and VII dangerously reactive? Consider, for example, the element sodium (Na), which is so reactive that it may burst into flames when exposed to air. The electron in the n=3 state has an unusually high energy. If we let a sodium atom come in contact with an oxygen atom, energy can be released by transferring the n=3 electron from the sodium to one of the vacant lower-energy n=2 states in the oxygen. This energy is transformed into heat. Any atom in column I is highly reactive for the same reason: it can release energy by giving away the electron that has an unusually high energy.
This shows that the columns relate to the filling of energy levels, but why does that have anything to do with chemistry? Why, for example, are the elements in columns I and VII dangerously reactive? Consider, for example, the element sodium (Na), which is so reactive that it may burst into flames when exposed to air. The electron in the n=3 state has an unusually high energy. If we let a sodium atom come in contact with an oxygen atom, energy can be released by transferring the n=3 electron from the sodium to one of the vacant lower-energy n=2 states in the oxygen. This energy is transformed into heat. Any atom in column I is highly reactive for the same reason: it can release energy by giving away the electron that has an unusually high energy.
Column VII is spectacularly reactive for the opposite reason: these atoms have a single vacancy in a low-energy state, so energy is released when these atoms steal an electron from another atom.
It might seem as though these arguments would only explain reactions of atoms that are in different rows of the periodic table, because only in these reactions can a transferred electron move from a higher-n state to a lower-n state. This is incorrect. An n=2 electron in fluorine (F), for example, would have a different energy than an n=2 electron in lithium (Li), due to the different number of protons and electrons with which it is interacting. Roughly speaking, the n=2 electron in fluorine is more tightly bound (lower in energy) because of the larger number of protons attracting it. The effect of the increased number of attracting protons is only partly counteracted by the increase in the number of repelling electrons, because the forces exerted on an electron by the other electrons are in many different directions and cancel out partially.
Example 1: Neutron stars
Here's an exotic example that doesn't even involve atoms. When a star runs out of fuel for its nuclear reactions, it begins to collapse under its own weight. Since Newton's law of gravity depends on the inverse square of the distance, the gravitational forces become stronger as the star collapses, which encourages it to collapse even further. The final result depends on the mass of the star, but let's consider a star that's only a little more massive than our own sun. Such a star will collapse to the point where the gravitational energy being released is sufficient to cause the reaction p+e-→n+ν to occur. (As you found in homework problem 10 on page 789, this reaction can only occur when there is some source of energy, because the mass-energy of the products is greater than the mass-energy of the things being consumed.) The neutrinos fly off and are never heard from again, so we're left with a star consisting only of neutrons!
Now the exclusion principle comes into play. The collapse can't continue indefinitely. The situation is in fact closely analogous to that of an atom. A lead atom's cloud of 82 electrons can't shrink down to the size of a hydrogen atom, because only two electrons can have the lowest-energy wave pattern. The same happens with the neutron star. No physical repulsion keeps the neutrons apart. They're electrically neutral, so they don't repel or attract one another electrically. The gravitational force is attractive, and as the collapse proceeds to the point where the neutrons come within range of the strong nuclear force (~10-15m), we even start getting nuclear attraction. The only thing that stops the whole process is the exclusion principle. The star ends up being only a few kilometers across, and has the same billion-ton-per-teaspoon density as an atomic nucleus. Indeed, we can think of it as one big nucleus (with atomic number zero, because there are no protons).
As with a spinning figure skater pulling her arms in, conservation of angular momentum makes the star spin faster and faster. The whole object may end up with a rotational period of a fraction of a second! Such a star sends out radio pulses with each revolution, like a sort of lighthouse. The first time such a signal was detected, radio astronomers thought that it was a signal from aliens.
36.6 Atoms with more than one electron by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.
Calculators and Collections
- Comments
- Attachments
- Stats
No comments |

