Tags | |
UUID | 4fd64f74-1ebd-11e6-9770-bc764e2038f2 |
pOH[1] is the measure of basicity of a solution. pOH measurement is derived from the measure of hydroxide ion concentration. Since the ion concentration is small in the solution, it is converted by the equation above to pOH (the equation above is a derivation of a equation that can be found here) and compared against a scale. pH scale ranges from 0 to 14, 0 being acidic and 14 being basic. At 25oC the concentration of each ion (H+ and OH-) is 1.0x10-7(mol/L), when both of the concentrations remain equal then the solution becomes neutral, which is a pH of 7 on the scale.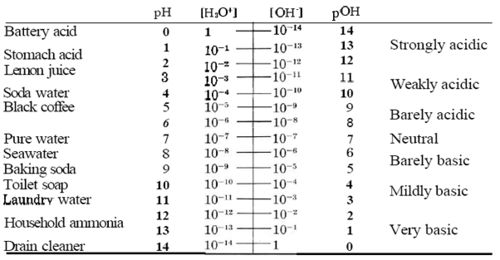
Description
The equation is
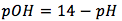 [2]
[2]
where
- pH is the acidity measurement (unit-less)
- pOH is the basicity measurement (unit-less)
- pKw is the natural log of the water equilibrium constant, which is always 14 (to learn more about pKw go here)
Related topics
Supplement Material
References
[1]https://en.wikipedia.org/wiki/PH#pOH
[2]Whitten, et al. 10th Edition. Pp.713
[pH diagram]http://wiki.chemprime.chemeddl.org/index.php/PH_and_pOH_in_Everyday_Life
- Comments
- Attachments
- Stats
No comments |
