Tags | |
UUID | 1acea466-1fad-11e6-9770-bc764e2038f2 |
Hydronium ion[1] (H3O+, also known as H+) and the hydroxide ion[2] (OH-) can be obtained from a known concentration of the other ion and the equation Kw=[H+][OH-]. This equation is known as equilibrium of water and originates from the acid-base reaction of water, also known as autoionization of water. Which occurs by the following process, as illustrated:
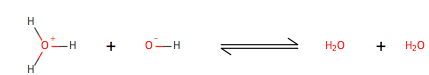
Two water molecules collide they exchange a proton, a positively charged particle, in this case it is a hydrogen missing an electron. One of the water molecules acts as an acid and the other acts as a base. There are three different acid-base theories. Bronsted-Lowery theory suggests that an acid donates a proton to the base, Lewis theory suggests that an acid accepts electrons from the base, and Arrhenius theory suggests that an acid is any molecule that increases the hydronium concentration in water and a base increases the concentration of a hydroxide. Since water autoionizes Arrhenius theory does not explain the formation of either the hydroxide or hydronium ions, which are known to be as conjugate base (OH-) and conjugate acid(H+). At 25oC the concentration of each ions is 1.0x10-7 (mol/L), when both of the concentrations remain equal then the solution becomes neutral.
pH[3] is the measure of acidity of a solution. pH measurement is derived from the measure of hydronium ion concentration.pOH[4] is a measure of basicity of a solution and its measurement is derived from the hydroxide ion concentration. Since the ion concentrations are small in the solution to pH and pOH, and then compared against a scale. pH scale ranges from 0 to 14, 0 being acidic and 14 being basic.
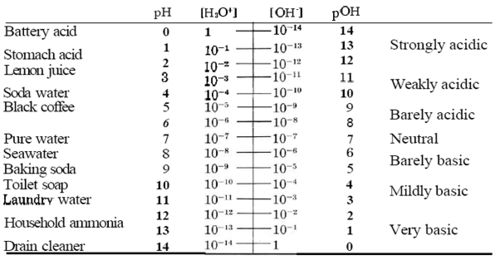
pKw[5] is the negative log of Kw, which is the equilibrium of water. Equilibrium of water is the multiplication of the hydronium ion(H+) and the hydroxide ion (OH-), equaling to 1.0x10-14. The negative log of Kw is 14, which is also the total of pH and pOH.
Supplement Material
- Khan Academy:
- CrashCourse:
- HyperPhysics:
- ChemWiki
References
[1]https://en.wikipedia.org/wiki/Hydronium
[2]https://en.wikipedia.org/wiki/Hydroxide
[3]https://en.wikipedia.org/wiki/PH
[4]https://en.wikipedia.org/wiki/PH#pOH
[5]https://en.wikipedia.org/wiki/Self-ionization_of_water
[pH scale diagram]http://wiki.chemprime.chemeddl.org/index.php/PH_and_pOH_in_Everyday_Life
Equations and Data Items
Collections
- Comments
- Attachments
- Stats
No comments |
