Standard Cell Potential
Tags | |
UUID | b7b3c834-1f95-11e6-9770-bc764e2038f2 |
The Standard Cell Potential Equation, Ecell0 = Ecathode0 + Eanode0, is based off of the potential energy per unit of charge of both the cathode and anode. Ecathode0 and Eanode0 are both values of reduction potential and are used to find the difference of the two electrodes, or the voltage of the cell. The voltage of the cell is calculated from the two half cells, as shown in the figure below. The inputs for this equation are the reduction potential values, as mentioned above. The default units are volts (V).
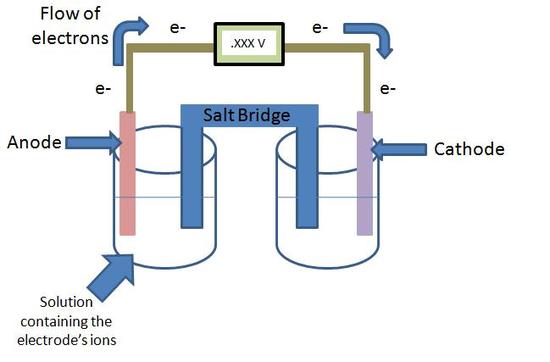 Electrochemical cell with voltmeter
Electrochemical cell with voltmeter
The cathode of an electrochemical cell is the metal that accepts electrons, whereas the anode donates the electrons. The common mnemonic device OIL RIG can be used here: "Oxidation Is Loss, Reduction Is Gain". The anode is losing electrons, so it is being oxidized, while the cathode is gaining electrons and is being reduced.
Uses
Standard Cell Potential is used to explain how much voltage is created between two half cells. This is used in batteries everywhere, from the battery in your car to the battery in your television remote control. This equation allows scientists to calculate how efficient a battery could be before physically testing it.
References
Collections
- Comments
- Attachments
- Stats
No comments |
