pOH (from [OH-])
pOH=−log([OH−])
Tags | |
UUID | 212cb741-1868-11e6-9770-bc764e2038f2 |
pOH[1] is the measure of basicity of a solution. pOH measurement is derived from the measure of hydroxide ion concentration. Since the ion concentration is small in the solution, it is converted by the equation above to pOH. pH scale ranges from 0 to 14, 0 being acidic and 14 being basic. At 25oC the concentration of each ion (H+ and OH-) is 1.0x10-7(mol/L), when both of the concentrations remain equal then the solution becomes neutral, which is a pH of 7 on the scale.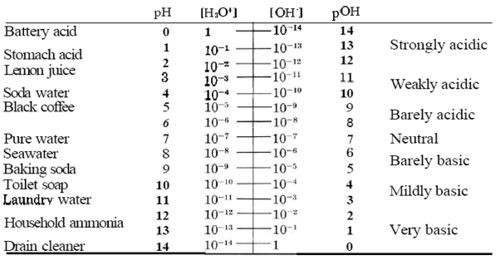
Description
The equation is
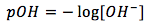 [2]
[2]
where
- pOH is the basicity measurement (unit-less)
- [OH-] is the hydroxide ion concentration in units of (mol/L)
Related Material
Supplement Material
References
[1]https://en.wikipedia.org/wiki/PH#pOH
[2]Whitten, et al. 10th Edition. Pp.713
[pH diagram]http://wiki.chemprime.chemeddl.org/index.php/PH_and_pOH_in_Everyday_Life
This equation, pOH (from [OH-]), is used in 6 pages
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.
