LM 0.05 Basics of the metric system Collection
Tags | |
UUID | 1e33e051-f145-11e9-8682-bc764e2038f2 |
0.5 Basics of the metric system by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.
0.5 Basics of the metric system
The metric system
Units were not standardized until fairly recently in history, so when the physicist Isaac Newton gave the result of an experiment with a pendulum, he had to specify not just that the string was 37 78 inches long but that it was "3778 London inches long.” The inch as defined in Yorkshire would have been different. Even after the British Empire standardized its units, it was still very inconvenient to do calculations involving money, volume, distance, time, or weight, because of all the odd conversion factors, like 16 ounces in a pound, and 5280 feet in a mile. Through the nineteenth century, schoolchildren squandered most of their mathematical education in preparing to do calculations such as making change when a customer in a shop offered a one-crown note for a book costing two pounds, thirteen shillings and tuppence. The dollar has always been decimal, and British money went decimal decades ago, but the United States is still saddled with the antiquated system of feet, inches, pounds, ounces and so on.
Every country in the world besides the U.S. uses a system of units known in English as the “metric system.2” This system is entirely decimal, thanks to the same eminently logical people who brought about the French Revolution. In deference to France, the system's official name is the Système International, or SI, meaning International System. (The phrase “SI system” is therefore redundant.)
The wonderful thing about the SI is that people who live in countries more modern than ours do not need to memorize how many ounces there are in a pound, how many cups in a pint, how many feet in a mile, etc. The whole system works with a single, consistent set of Greek and Latin prefixes that modify the basic units. Each prefix stands for a power of ten, and has an abbreviation that can be combined with the symbol for the unit. For instance, the meter is a unit of distance. The prefix kilo- stands for 103, so a kilometer, 1 km, is a thousand meters.
The basic units of the metric system are the meter for distance, the second for time, and the gram for mass.
The following are the most common metric prefixes. You should memorize them.
| prefix | meaning | example | ||
| kilo- | k | 103 | 60 kg | a person’s mass |
|---|---|---|---|---|
| centi- | c | 10 -2 | 28 cm | height of a piece of paper |
| milli- | m | 10 -3 | 1 ms | time for one vibration of a guitar string playing the note D |
The prefix centi-, meaning 10-2, is only used in the centimeter; a hundredth of a gram would not be written as 1 cg but as 10 mg. The centi- prefix can be easily remembered because a cent is 10-2 dollars. The official SI abbreviation for seconds is “s” (not “sec”) and grams are “g” (not “gm”).
The second
When I stated briefly above that the second was a unit of time, it may not have occurred to you that this was not much of a definition. We can make a dictionary-style definition of a term like “time,” or give a general description like Isaac Newton's: “Absolute, true, and mathematical time, of itself, and from its own nature, flows equably without relation to anything external...” Newton's characterization sounds impressive, but physicists today would consider it useless as a definition of time. Today, the physical sciences are based on operational definitions, which means definitions that spell out the actual steps (operations) required to measure something numerically.
In an era when our toasters, pens, and coffee pots tell us the time, it is far from obvious to most people what is the fundamental operational definition of time. Until recently, the hour, minute, and second were defined operationally in terms of the time required for the earth to rotate about its axis. Unfortunately, the Earth's rotation is slowing down slightly, and by 1967 this was becoming an issue in scientific experiments requiring precise time measurements. The second was therefore redefined as the time required for a certain number of vibrations of the light waves emitted by a cesium atoms in a lamp constructed like a familiar neon sign but with the neon replaced by cesium. The new definition not only promises to stay constant indefinitely, but for scientists is a more convenient way of calibrating a clock than having to carry out astronomical measurements.
self-check: What is a possible operational definition of how strong a person is?
(answer in the back of the PDF version of the book)
The meter
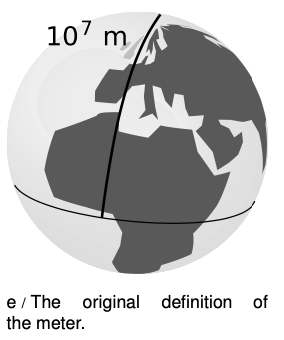 The French originally defined the meter as 10-7 times the distance from the equator to the north pole, as measured through Paris (of course). Even if the definition was operational, the operation of traveling to the north pole and laying a surveying chain behind you was not one that most working scientists wanted to carry out. Fairly soon, a standard was created in the form of a metal bar with two scratches on it. This was replaced by an atomic standard in 1960, and finally in 1983 by the current definition, which is that the meter is the distance traveled by light in a vacuum over a period of (1/299792458) seconds.
The French originally defined the meter as 10-7 times the distance from the equator to the north pole, as measured through Paris (of course). Even if the definition was operational, the operation of traveling to the north pole and laying a surveying chain behind you was not one that most working scientists wanted to carry out. Fairly soon, a standard was created in the form of a metal bar with two scratches on it. This was replaced by an atomic standard in 1960, and finally in 1983 by the current definition, which is that the meter is the distance traveled by light in a vacuum over a period of (1/299792458) seconds.
The kilogram
The third base unit of the SI is the kilogram, a unit of mass. Mass is intended to be a measure of the amount of a substance, but that is not an operational definition. Bathroom scales work by measuring our planet's gravitational attraction for the object being weighed, but using that type of scale to define mass operationally would be undesirable because gravity varies in strength from place to place on the earth.
 There's a surprising amount of disagreement among physics textbooks about how mass should be defined, but here's how it's actually handled by the few working physicists who specialize in ultra-high-precision measurements. They maintain a physical object in Paris, which is the standard kilogram, a cylinder made of platinum-iridium alloy. Duplicates are checked against this mother of all kilograms by putting the original and the copy on the two opposite pans of a balance. Although this method of comparison depends on gravity, the problems associated with differences in gravity in different geographical locations are bypassed, because the two objects are being compared in the same place. The duplicates can then be removed from the Parisian kilogram shrine and transported elsewhere in the world. It would be desirable to replace this at some point with a universally accessible atomic standard rather than one based on a specific artifact, but as of 2010 the technology for automated counting of large numbers of atoms has not gotten good enough to make that work with the desired precision.
There's a surprising amount of disagreement among physics textbooks about how mass should be defined, but here's how it's actually handled by the few working physicists who specialize in ultra-high-precision measurements. They maintain a physical object in Paris, which is the standard kilogram, a cylinder made of platinum-iridium alloy. Duplicates are checked against this mother of all kilograms by putting the original and the copy on the two opposite pans of a balance. Although this method of comparison depends on gravity, the problems associated with differences in gravity in different geographical locations are bypassed, because the two objects are being compared in the same place. The duplicates can then be removed from the Parisian kilogram shrine and transported elsewhere in the world. It would be desirable to replace this at some point with a universally accessible atomic standard rather than one based on a specific artifact, but as of 2010 the technology for automated counting of large numbers of atoms has not gotten good enough to make that work with the desired precision.
Combinations of metric units
Just about anything you want to measure can be measured with some combination of meters, kilograms, and seconds. Speed can be measured in m/s, volume in m3, and density in kg/m3. Part of what makes the SI great is this basic simplicity. No more funny units like a cord of wood, a bolt of cloth, or a jigger of whiskey. No more liquid and dry measure. Just a simple, consistent set of units. The SI measures put together from meters, kilograms, and seconds make up the mks system. For example, the mks unit of speed is m/s, not km/hr.
Checking units
A useful technique for finding mistakes in one's algebra is to analyze the units associated with the variables.
Example 1: Checking units
⇒ Jae starts from the formula V=13Ah for the volume of a cone, where A is the area of its base, and h is its height. He wants to find an equation that will tell him how tall a conical tent has to be in order to have a certain volume, given its radius. His algebra goes like this:
[1] V=13Ah
[2] A=πr2
[3] V=13πr2h
[4] h=πr2/3V
Is his algebra correct? If not, find the mistake.
⇒ Line 4 is supposed to be an equation for the height, so the units of the expression on the right-hand side had better equal meters. The pi and the 3 are unitless, so we can ignore them. In terms of units, line 4 becomes
m=m2m3=1m.
This is false, so there must be a mistake in the algebra. The units of lines 1, 2, and 3 check out, so the mistake must be in the step from line 3 to line 4. In fact the result should have been
Now the units check: m=m3m2.
Discussion Question
A Isaac Newton wrote, “... the natural days are truly unequal, though they are commonly considered as equal, and used for a measure of time... It may be that there is no such thing as an equable motion, whereby time may be accurately measured. All motions may be accelerated or retarded...” Newton was right. Even the modern definition of the second in terms of light emitted by cesium atoms is subject to variation. For instance, magnetic fields could cause the cesium atoms to emit light with a slightly different rate of vibration. What makes us think, though, that a pendulum clock is more accurate than a sundial, or that a cesium atom is a more accurate timekeeper than a pendulum clock? That is, how can one test experimentally how the accuracies of different time standards compare?
0.5 Basics of the metric system by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.
Equations
- Cone - Volume f(A,h) KurtHeckman Use Equation
- Area of a Circle KurtHeckman Use Equation
- Cone - Height KurtHeckman Use Equation
- Cone - Volume KurtHeckman Use Equation
- Comments
- Attachments
- Stats
No comments |
