pH (from [OH-])
Tags | |
UUID | e783043d-1ec1-11e6-9770-bc764e2038f2 |
The pH of a Concentration of Hydroxide [OH-] calculator computes the pH of a concentration of Hydroxide.
INSTRUCTIONS: Choose units and enter the following:
- [OH-] Concentration of Hydroxide
acidity (pH): The calculator returns the pH as a real number.
The Math / Science
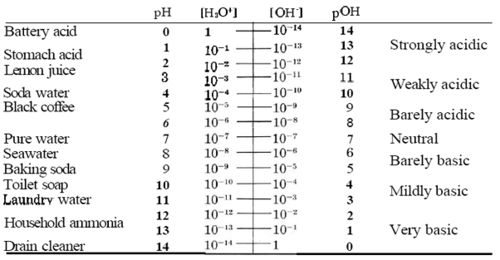
pH[1] is the measure of acidity of a solution. pH measurement is derived from the measure of hydronium ion concentration. Since the ion concentration is small in the solution, it is converted by the equation above to pH (the equation below is a derivation of a equation that can be found here) and compared against a scale/ pH scale ranges from 0 to 14, 0 being acidic and 14 being basic. At 25oC the concentration of each ion (H+ and OH-) is 1.0x10-7 (mol/L), when both of the concentrations remain equal then the solution becomes neutral, which is a pH of 7 on the scale.
Hydroxide (OH-) is molecule containing a hydrogen and oxygen atom held together in a covalent bond. Hydroxide is commonly used for several purposes medicine (kidney stone prevention and blood preservative) and as a food preservative. The molecular weight of hydroxide is 17.007 grams per mole.
The formula for the pH of a concentration of hydroxide is:
pH = 14 + log[OH-]
where:
- [OH-] is the hydroxide ion concentration in units of (mol/L)
- pH is acidity measurement
Related Topics
Supplement Material
- Khan Academy:
- HyperPhysics:pH
References
[1]https://en.wikipedia.org/wiki/PH
[2]Whitten, et al. 10th Edition. Pp.713
[pH scale diagram]http://wiki.chemprime.chemeddl.org/index.php/PH_and_pOH_in_Everyday_Life
Collections
- Comments
- Attachments
- Stats
No comments |
