CHM1 11 Molality and Mole Fractions Collection
Molality and Mole Fractions
From ChemPRIME
Molality
The molality (m) of a solution is used to represent the amount of moles of solute per kilogram of the solvent.
m=Moles of SoluteKilograms of Solventm=Moles of SoluteKilograms of Solvent
The molarity and molality equations differ only from their denominators. However, this is a huge difference. As you may remember, volume varies with different temperatures. At higher temperatures, volumes of liquids increase, while at lower temperatures, volumes of liquids decrease. Therefore, molarities of solutions also vary at different temperatures. This creates an advantage for using molality over molarity. Using molalities rather than molarities for lab experiments would best keep the results within a closer range. Because volume is not a part of its equation, it makes molality independent of temperature.
EXAMPLE 4 0.88g NaCl is dissolved in 2.0L water. Find its molality.
Mass and Mole Fractions
When speaking of solubility or miscibility, or when doing quantitative experiments involving solutions, it is necessary to know the exact composition of a solution. This is invariably given in terms of a ratio telling us how much solute is dissolved in a unit quantity of solvent or solution. The ratio can be a ratio of masses, of amounts of substances, or of volumes, or it can be some combination of these. For example, concentration was defined before as the amount of solute per unit volume of solution:
Csolute=nsoluteVsolutionCsolute=nsoluteVsolution
The two simplest measures of the composition are the mass fraction w, which is the ratio of the mass of solute to the total mass of solution, and the mole fraction x, which is the ratio of the amount of solute to the total amount of substance in the solution. If we indicate the solute by A and the solvent by B, the mass fraction and the mole fraction are defined by
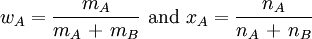
EXAMPLE 5 A solution is prepared by dissolving 18.65 g naphthalene, C10H8 in 89.32 g benzene, C6H6. Find (a) the mass fraction and (b) the mole fraction of the naphthalene.
Equations
- Mole fraction Dasha Use Equation
- Mass Fraction Dasha Use Equation
- Molality DavidC Use Equation
- Molarity ekskekel Use Equation
- Comments
- Attachments
- Stats
No comments |
