What is a titration?
A titration is an analytical method of determining a concentration of an unknown solution (known as analyte) by reacting with a solution with a known concentration (known as titrant). Since we want a reaction to occur a titration is done via acid-base reaction. An indicator aids in the process of titration, specifically indicating when the acid and the base have reacted. However, in our case we are building the titration curve which helps determine at which pH and added volume of strong base the equivalence point is going to occur.
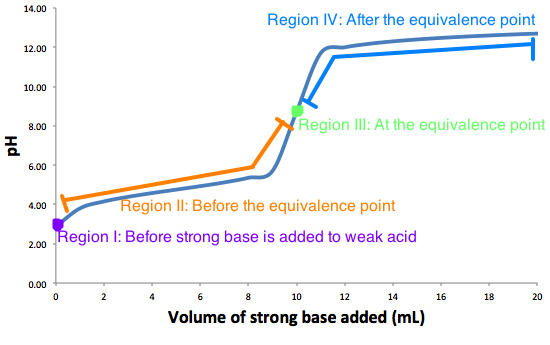
The graph represents a typical weak acid-strong base titration curve with all 4 regions labeled.
How is a titration done?
The most common titration method is done with an addition of an indicator. A titrant, in our case happens to be the strong base, is filled in to a 50mL buret. The analyte, the weak acid, is placed in to a beaker underneath the buret . An indicator is also added to the analyte, and changes from clear to a color as a sign that the acid and the base have reacted fully, thus indicating that the end point (or the equivalence point) has been reached. There are many different types of indicators and the one to use depends on the analyte.
Figure 1. Titration method with an indicator.
Another method of titration is done with a pH meter. A titrant is once again added to the 50mL buret and the analyte is placed in to a beaker underneath. A pH meter is an instrument that is then placed in to the analyte and reads out the pH of the solution as the titrant is added. The end point is reached when the pH meter reads a pH of 7, which indicates that the acid and the base have fully reacted and the solution is now neutral.
To see how a titration is done with a pH meter check out this video.
Addition Information
References
Harris, 9th Edition. Pp.236-238
