The Second -order Rate Law (Integral Form) calculator computes the second order rate (concentration) based on the concentration of substance, rate the constant and duration of time.
INSTRUCTIONS: Choose units and enter the following:
- [A]0 Initial Concentration of Substance
- (k) Rate Constant (L/ (mol⋅s))
- (t) Duration of Reaction
Concentration [A]t: The calculator returns the concentration in moles per liter. However this can be automatically converted to compatible units via the pull-down menu.
The Math / Science
The second-order rate law[1] equation, specifically the integral form looks at the concentration of the reactants at a certain point in time. The integral form of the equation was obtained from the differential form and the full integration can be found here. Unlike the first-order rate law, the second-order depends on two reactants and thus there are three different cases:
Case 1. Both of the reactants are of the same substance A
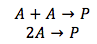
Since both of the reactants are the same, the concentration is the same for both of the reactants. The integrated rate equation is as show above.
Case 2. The reactants are of two different substances (A and B) and their concentrations are different
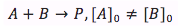
When both of the reactants are different and have different concentrations the differential equation is integrated differently giving the equation
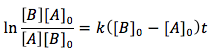
Case 3. The reactants are of two different substance (A and B) but their concentrations are the same.
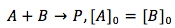 Since the concentrations are the same, the substances can be treated as one and the integration becomes just like in case 1.
Since the concentrations are the same, the substances can be treated as one and the integration becomes just like in case 1.
Second-order rate law (integral form) Formula
The Second-order rate law (integral form) equation is
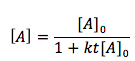 [2]
[2]
where
- [A] is the end concentration in moles per liter (mol/L)
- [A]0 is the initial concentration of A in units of (mol/L)
- t is the time or the duration of the reaction in units of (sec)
- k is the rate law constant in units of (L/mol* sec)
- [A] is the concentration of substance A at a certain time during the reaction in units of (mol/L)
Related Topics
Chemistry Rate Law Calculators
- Zero Order Rate Law (Integral form)
- Zero Order Half Life
- Zero Order Rate Law
- First Order Rate Law (Integral form)
- First Order Half Life
- First Order Rate Law
- Second Order Rate Law (Integral form)
- Second Order Half Life
- Second Order Rate Law
Supplement Material
- Khan Academy: Rate law and reaction order
References
[1]https://en.wikipedia.org/wiki/Rate_equation
[2]Whitten, et al. 10th Edition. Pp. 626,629,631
