Chemistry
vCalc's Chemistry library contains information that goes with Chemistry formulas (e.g. PV=nRT) and data constants (e.g. Gas Constant - R) related to the properties and composition of matter. Some of the most common formulas are based on well known laws: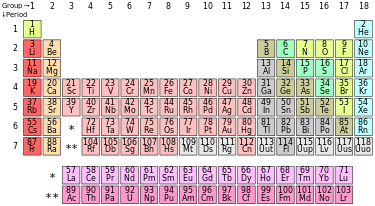
PERIODIC TABLE INFORMATION
by: Element Name or Atomic Number
- Ideal Gas Law: P•V = n•R•T
- Charles Law: `V_1`•`T_2` = `V_2`•`T_1`
- Boyles Law: `P_1`• `V_1` = `P_2` •`V_2`
- Gay-Lussac Law: `T_1` • `P_2` = `T_2` • `P_1`
- Combined Gas Law: P•V / T = k
Chemistry is the physical science which examines the composition, structure, properties and behavior of matter. It focuses on atoms, their interactions with other atoms and phenomena such as the forming of chemical bonds between atoms to create chemical compounds. In chemistry, matter is defined as anything that has rest mass and volume (it takes up space) and is made up of particles.
Parent Categories
Subcategories
Chemistry Calculators and Collections
- Base Fundamental Units Conversions
- Boyle's Law
- Boyle's Law Calculator
- Bragg's Law
- CHM1 11 Boiling Point Elevation and Freezing Point Depression Co
- CHM1 11 Colligative Properties and Dissociation of Electrolytes
- CHM1 11 Colloids Collection
- CHM1 11 Dissolution of Gases in Liquids Collection
- CHM1 11 Dissolution of Liquids in Liquids Collection
- CHM1 11 Dissolution of Solids in Liquids Collection
- CHM1 11 Effects of Temperature and Pressure on Solubility Collec
- CHM1 11 End of Chapter Problems Collection
- CHM1 11 Fractional Distillation Collection
- CHM1 11 Molality and Mole Fractions Collection
- CHM1 11 Molecular Weight by BP Elevation and FP Depression Colle
- CHM1 11 Osmotic Pressure Collection
- CHM1 11 Rates of Dissociation and Saturation Collection
- CHM1 11 Solubility Collection
- CHM1 11 Vapor Pressure Collection
- CHM1 12 Bond Energies Collection
- CHM1 12 Calorimetry Collection
- CHM1 12 Dispersal of Energy and Matter Collection
- CHM1 12 End-of-Chapter Problems Collection
- CHM1 12 Enthalpy Collection
- CHM1 12 Enthalpy and Internal Energy Collection
- CHM1 12 Entropy Collection
- CHM1 12 Free Energy Collection
- CHM1 12 Hess's Law Collection
- CHM1 12 Internal Energy Collection
- CHM1 12 Molar Enthalpies of Formation Collection
- CHM1 12 Spontaneity Collection
- CHM1 12 Standard States Collection
- CHM1 12 Temperature Dependence of Free Energy Collection
- CHM1 12 Thermochemical Equations Collection
- CHM1 12 Thermodynamics Terms and Conventions Collection
- CHM1 12 Thermodynamics and the 1st Law Collection
- CHM1 13 Catalysts Collection
- CHM1 13 Collision Theory of Reaction Rates Collection
- CHM1 13 Concentration of Reactants: Rate-Law Expressions Collect
- CHM1 13 End-of-Chapter Problems Collection
- CHM1 13 Introduction Collection
- CHM1 13 Reaction Mechanisms Collection
- CHM1 13 Temperature Collection
- CHM1 13 The Integrated Rate Equations Collection
- CHM1 13 The Rate of a Reaction Collection Collection
- CHM1 14 Amphoterism Collection
- CHM1 14 Autoionization of Water Collection
- CHM1 14 Conjugate Acid-Base Pairs Collection
- CHM1 14 End-of-Chapter Problems Collection
- CHM1 14 Example 2 Collection
- CHM1 14 Example 3 Collection
- CHM1 14 Example 4 Collection
- CHM1 14 Example 5 Collection
- CHM1 14 Example 7 Collection
- CHM1 14 Hydronium and Hydroxide Ions Collection
- CHM1 14 Introduction Collection
- CHM1 14 Lewis Theory Collection
- CHM1 14 Strong Acids and Bases Collection
- CHM1 14 The Arrhenius Theory Collection
- CHM1 14 The Bronsted-Lowry Theory Collection
- CHM1 14 Weak Acids and Bases Collection
- CHM1 14Example1 Collection
- CHM1 15 End-of-Chapter Problems Collection
- CHM1 15 Example 1 Collection
- CHM1 15 Example 2 Collection
- CHM1 15 Molarity Tables and Calculations Collection
- CHM1 15 Titration Calculations Collection
- CHM1 15 Titrations Collection
- CHM1 16 Addition of a Catalyst Collection
- CHM1 16 Answers to End-of-Chapter Problems Collection
- CHM1 16 Calculations with the Equilibrium Constant Collection
- CHM1 16 Changes in Concentration Collection
- CHM1 16 Changes in Temperature Collection
- CHM1 16 Changes in Volume and Pressure Collection
- CHM1 16 Disturbing a System at Equilibrium Collection
- CHM1 16 End-of-Chapter Problems Collection
- CHM1 16 Equilibrium Constant Collection
- CHM1 16 Example 2 Collection
- CHM1 16 Example 3 Collection
- CHM1 16 Example 6 Collection
- CHM1 16 Gibbs Free Energy and the Equilibrium Constant Collectio
- CHM1 16 Haber Process Collection
- CHM1 16 Introduction Collection
- CHM1 16 Reaction Quotient Collection
- CHM1 16 Van't Hoff Equation Collection
- CHM1 17 Qualitative Description of Acid–Base Equilibriums Collec
- CHM1 17 pH and pOH Collection
- CHM1 Acid-Base Rxns Collection
- CHM1 Index Collection
- Calculadora Para La Ley de Boyle
- Chemistry 101
- Chemistry Calculator
- General Chemistry Collection
- LM 26.1 Atoms Collection
- LM 26.4 The nucleus Collection
- LM 33.4 Exponential decay and half-life Collection
- LM 33.5 Applications of calculus Collection
- La Ley de Bragg
- Measurement of Concentration
- Rate Law
- Titration of weak acid with a strong base
Chemistry Equations
- Absorbance Use Equation
- Absorbance Use Equation
- Absorption (from EBC) Use Equation
- Acentric Factor Use Equation
- Acentric Factor (`p^"sat", p_c`) Use Equation
- Activation Energy Use Equation
- Adjusted Retention time Use Equation
- Amount of Water and Anhydrous Compound Calculator Use Equation
- Antes del Punto de Equivalencia Use Equation
- Antes que se Añade una Base Fuerte a un ácido débil Use Equation
- Arrhenius Activation Energy for Two Temperature Use Equation
- Arrhenius Equation Use Equation
- Atomic Mass Deficiency Use Equation
- Atomic Weight Use Equation
- Atomic Weight of Soil Nutrients Use Equation
- Balance de Carga Use Equation
- Balance de Masa Use Equation
- Balmer-Rydberg Equation Use Equation
- Beer's Law Use Equation
- Boyles Law (final pressure) Use Equation
- Boyles Law (final volume) Use Equation
- Boyles Law (initial pressure) Use Equation
- Boyles Law (initial volume) Use Equation
- Calculadora para encontrar pOH con pH y pKw Use Equation
- Cambio de energía libre de Gibbs (presión/temperatura constante) Use Equation
- Capacitance Unit Conversion Use Equation
- Charge balance Use Equation
- Charles Law (Final temperature) Use Equation
- Charles Law (Initial temperature) Use Equation
- Clausius-Clapeyron Equation Use Equation
- Clausius-Clapeyron Temperature Use Equation
- Combined Gas Law (final pressure) Use Equation
- Combined Gas Law (final temp) Use Equation
- Combined Gas Law (final volume) Use Equation
- Combined Gas Law (k) Use Equation
- Combined Gas Law (P) Use Equation
- Combined Gas Law (T) Use Equation
- Combined Gas Law (V) Use Equation
- Compressibility Factor Use Equation
- Concentration Use Equation
- Conversion from Mass to Moles Use Equation
- Convert p-functions values to Molar Concentrations Use Equation
- Cottrell Use Equation
- Coulomb's law Use Equation
- Coulombs Law Use Equation
- Dalton's Law of Partial Pressures Use Equation
- De Broglie Equation Use Equation
- DeBroglie Wavelength Use Equation
- DeBroglie wavelength (relativistic) Use Equation
- Density Use Equation
- Density of a Liquid Use Equation
- Density of a Liquid Use Equation
- Density of Air-free Water Use Equation
- Diffusion Coefficient of Liquid Phase Use Equation
- Dilution Factor Use Equation
- Dipole Moment Use Equation
- Disociación de fracciones de un ácido Use Equation
- Ebullioscopic constant Use Equation
- Ecuación extendida de debye-huckel Use Equation
- Ecuación para bases débiles Use Equation
- Ecuación para un ácido débil Use Equation
- Electric Current Unit Conversion Use Equation
- Electric Field Intensity Unit Conversion Use Equation
- Electrostatic Potential Energy Use Equation
- Energía del Fotón Use Equation
- Energy of Light Use Equation
- Entropy Law Use Equation
- Equation for weak acid Use Equation
- Equation for weak base Use Equation
- Equimolar Counter-Diffusion (Gases) Use Equation
- Extended debye-huckle equation Use Equation
- Factor de Retención Use Equation
- Femtometer-to-Meter Use Equation
- Fick's Law (one dimensional diffusion with time) Use Equation
- Find the number of moles in a solute Use Equation
- Force Unit Conversion Use Equation
- Fraction dissociation for weak base Use Equation
- Fraction dissociation of an acid Use Equation
- Fuerza iónica Use Equation
- Fugacity of component i in Liquid phase Use Equation
- Fugacity of component i in Vapor phase Use Equation
- Gay-Lussac Law (final pressure) Use Equation
- Gay-Lussac Law (final temperature) Use Equation
- Gay-Lussac Law (initial pressure) Use Equation
- Gay-Lussac Law (initial temperature) Use Equation
- Gibbs Free Energy Change (constant pressure and temperature) Use Equation
- Grahams Law of Diffusion (Effusion Rate) Use Equation
- Grahams Law of Diffusion (Molar Mass) Use Equation
- Half-life of Decay Use Equation
- Hess's Law Use Equation
- How many grams of Element are in a Molecule sample Use Equation
- Ideal gas law (molar form) Use Equation
- Ideal gas law (pressure) Use Equation
- Ideal gas law (temperature) Use Equation
- Ideal gas law (volume) Use Equation
- Ideal gas law (volume) Use Equation
- Incertidumbre en la multiplicación y división Use Equation
- Incertidumbre en suma y resta Use Equation
- Incertidumbre relativa Use Equation
- Inherent Viscosity Use Equation
- Inherent Viscosity[m_i,V, eta, et_r] Use Equation
- Ionic strength Use Equation
- Isotopes Relative Atomic Mass (RAM) Use Equation
- kPa to mmHg Use Equation
- K_a (Chemical Equilibrium Constant ) Use Equation
- La Absorbancia Use Equation
- La diferencia de energía libre y el potencial eléctrico Use Equation
- La Ecuacion de Balmer-Rydberg Use Equation
- La Ecuacion de Clausius-Clapeyron Use Equation
- La ecuación de van Deemter Use Equation
- La Ley de Beer-Lambert Use Equation
- La Ley de Boyle (el volumen final) Use Equation
- La Ley de Boyle (el volumen inicial) Use Equation
- La Ley de Boyle (la presion final) Use Equation
- La Ley de Boyle (la presion inicial ) Use Equation
- La Ley de Charles (la temperatura final) Use Equation
- La Ley de Charles (La temperatura inicial) Use Equation
- La Ley de Coulomb Use Equation
- La Ley de Gas Combinada (el volumen) Use Equation
- La Ley de Gas Combinada (la constante k) Use Equation
- La Ley de Gas Combinada (la presion) Use Equation
- La Ley de Gas Combinada (la temperatura) Use Equation
- La Ley de Gay-Lussac (la presion final) Use Equation
- La Ley de Gay-Lussac (la presion inicial) Use Equation
- La Ley de Gay-Lussac (la temperatura final) Use Equation
- La Ley de Gay-Lussac (la temperatura inicial) Use Equation
- La Ley de Hess Use Equation
- La Transmitancia Use Equation
- Lineweaver-Burk equation Use Equation
- MASS (G) Use Equation
- Mass balance Use Equation
- Mass Concentration Use Equation
- Mass Flow Rate Unit Conversion Use Equation
- Mass Fraction of Vapor Phase Use Equation
- Mass of an atom Use Equation
- Membrane Flux - when diffusion equation is known Use Equation
- Michaelis-Menton Equation Use Equation
- Molalidad Use Equation
- Molality Use Equation
- Molar Mass (Molar Weight) Use Equation
- Molar Mass Unit Conversion Use Equation
- Molar Weight Use Equation
- Molaridad Use Equation
- Molarity Use Equation
- Mole fraction Use Equation
- Molecular Diffusion (Gas) Use Equation
- Molecular Diffusion (Liquid) Use Equation
- Molecular Kinetic Energy Use Equation
- Moles of Titration Use Equation
- Nuclear Binding Energy Use Equation
- p-function Use Equation
- Paschen's Law Use Equation
- Peng-Robinson Equation of State Use Equation
- Percent by Mass (Weight Percent) Use Equation
- Percent relative uncertainty Use Equation
- Periodic Table Use Equation
- Periodic Table of Elements - details Use Equation
- Permiability Use Equation
- Peso Molar Use Equation
- Photon energy Use Equation
- pOH (using pH and pKw) Use Equation
- Porcentaje de incertidumbre relativa Use Equation
- Power Unit Conversion Use Equation
- Pressure Units Conversion Use Equation
- Products' Standard Enthalpy of Formation Use Equation
- Pure component Fugacity Use Equation
- Quantum Energy Use Equation
- Radiation Dosage Unit Conversion Use Equation
- Rate of Decay Use Equation
- Reactants' Standard Enthalpy of Formation Use Equation
- Reaction Quotient (Q) Use Equation
- Redlich-Kwong (a - constant) Use Equation
- Redlich-Kwong (b - constant) Use Equation
- Redlich-Kwong (pressure) Use Equation
- Region I : Before strong base is added to weak acid Use Equation
- Region II: Before the equivalence point Use Equation
- Region III : At the equivalence point Use Equation
- Region IV: After the equivalence point Use Equation
- Relación entre carga y moles Use Equation
- Relación entre trabajo y voltaje Use Equation
- Relation between charge and moles Use Equation
- Relation between free energy difference and electric potential Use Equation
- Relation between work and voltage Use Equation
- Relative Uncertainty Use Equation
- Relative Viscosity Use Equation
- Retention Factor Use Equation
- Rydberg Equation Use Equation
- Sackur-Tetrode equation Use Equation
- Soave mod. Redlich-Kwong Use Equation
- Standard Enthalpy of Reaction Use Equation
- Standard Enthalpy of Reaction [`v_i, Hf_i`] Use Equation
- Standard Gibbs Energy change of reaction `Delta G^0` Use Equation
- Temperatura en Equilibrio Use Equation
- Temperature at Equilibrium Use Equation
- Temperature Coefficient Q10 Use Equation
- Temperature Unit Conversion Use Equation
- Tiempo de Retención Ajustada Use Equation
- Titulación: Reacción en Punto de Equivalencia Use Equation
- Transmittance Use Equation
- Uncertainty in addition and subtraction Use Equation
- Uncertainty in multiplication and division Use Equation
- Unidirectional Diffusion (Gas) Use Equation
- van Deemter Equation Use Equation
- van der Waals (pressure) Use Equation
- van der Waals (temperature) Use Equation
- van der Waals Equation (pressure) Use Equation
- Velocidad de Desintegracion Use Equation
- Volume at STP Use Equation
- Volume percent Use Equation
- Volume Unit Conversion Use Equation
- vVolume Flow Rate Use Equation
- Weight percent Use Equation
- Weight/Volume percent Use Equation
- What is molarity of X in solution of XYZ Use Equation
- `K_a` (fugacity) Use Equation
- `K_a` (pressure, Ideal gas) Use Equation
Chemistry Constants
- Avogadro's number with units Use Constant
- Boltzmann Constant Use Constant
- Carbon Molar Mass Use Constant
- Coulomb's Constant (with Units) Use Constant
- deuteron molar mass Use Constant
- electron molar mass (chemistry) Use Constant
- Faraday constant Use Constant
- helion molar mass (chemistry) Use Constant
- Helium Molar Mass Use Constant
- Hydrogen Molar Mass Use Constant
- La Constante de Gas R Use Constant
- molar mass constant Use Constant
- Molar Mass Dry Air (SI Units) Use Constant
- Molar Mass of Dry Air Use Constant
- muon molar mass (chemistry) Use Constant
- neutron molar mass (chemistry) Use Constant
- Oxygen Molar Mass Use Constant
- Percentage Argon in Earth's Atmosphere Use Constant
- Percentage Carbon Dioxide in Earth's Atmosphere Use Constant
- Percentage Nitrogen in Earth's Atmosphere Use Constant
- Percentage Oxygen in Earth's Atmosphere Use Constant
- Percentage Oxygen in Earth's Atmosphere (Decimal) Use Constant
- pKa of 2-Aminobiphenyl Use Constant
- pKa of 2-Heptylamine Use Constant
- pKa of 2-Naphthylamine Use Constant
- pKa of 2-Propanamine Use Constant
- pKa of 4-Aminobiphenyl Use Constant
- pKa of 4-Benzylaniline Use Constant
- pKa of Allylamine Use Constant
- pKa of Aniline Use Constant
- pKa of Butylamine Use Constant
- pKa of Cyclohexylamine Use Constant
- pKa of Dibutylamine Use Constant
- pKa of Diethylamine Use Constant
- pKa of Dimethylamine Use Constant
- pKa of Diphenylamine Use Constant
- pKa of iso-Butylamine Use Constant
- pKa of m-Toluidine Use Constant
- pKa of n-Allylaniline Use Constant
- pKa of o-Toluidine Use Constant
- pKa of p-Toluidine Use Constant
- pKa of sec-Butylamine Use Constant
- pKa of tert-Butylamine Use Constant
- pKa of Triethylamine Use Constant
- pKa of Trimethylamine Use Constant
- proton molar mass (chemsitry) Use Constant
- R - Gas Constant (liter*atm / mol*K) Use Constant
- R - Gas Constant (SI units) Use Constant
- Rydberg constant Use Constant
- tau molar mass Use Constant
- triton molar mass Use Constant
- Attachments
No attachments |

