The Osmotic Pressure calculator computes the osmotic pressure based on the molar concentration of the solution (M), the temperature (T) and the Ideal Gas Constant (R).
INSTRUCTIONS: Choose units and enter the following:
- (M) molar concentration of the solution
- (T) temperature
Osmotic Pressure (Π): The pressure is returned in atmospheres. However, this can be automatically converted to compatible units via the pull-down menu.
The Math / Science
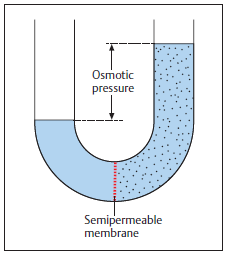
The Osmotic Pressure formula, Π = M•R•T, represents the pressure required to prevent a pure solvent from passing into a solution through osmosis, as shown in the figure below. Osmotic pressure plays an important role in cell regulation. It helps cells prevent too much water from either escaping or entering the cell, thus maintaining a homeostasis. Osmotic pressure can also be used to estimate molecular weights, particularly for higher weight molecules.
The Osmotic Pressure formula is:
Π = M•R•T
where:
- Π = Osmotic Pressure
- M = molar concentration of the solution
- R = ideal gas constant
- T = temperature
The default units are moles/liter for molar concentration and Kelvin for temperature.
Description
Osmotic pressure increases with temperature because temperature affects the number of solvent-membrane collisions per minute. It also increases with molarity because molarity leads to a higher or lower number of molecules hitting the membrane. A higher molarity leads to a stronger drive to equalize the concentration and increase disorder in the solution.
Supplemental Materials
Khan Academy - Diffusion and Osmosis

Water Related Calculators
- Underwater Pressure: Computes the added pressure exerted underwater by the water column directly overhead as a function of the density of the water, depth under water, and the acceleration due to gravity.
- Pore Water Pressure: Measure of the pressure of groundwater held within a soil or rock, in gaps between particles (pores), relative to atmospheric pressure.
- Osmotic Pressure: Computes the osmotic pressure based on the molar concentration of the solution (M), the temperature (T) and the Ideal Gas Constant (R).
- Density of water at STP: 998.2071 kg / m3
- Density of Deawater: 1,025 kg / m3
- Saltwater Intrusion: Uses the Ghyben-Herzberg Relation to compute the depth of fresh water in an aquifer below sea leve based on the depth of fresh water above sea level.
- Snow or Water Density: Returns the kilograms per meters cubed (kg/m3) for snow of different types or water at different temperatures per the US Geological Survey (USGS).
- Water Density by Temperature: Computes the density of water as a function of temperature, using the standard density of water (ρ) at standard temperature and pressure, and the unique temperature expansion coefficient of water.
- Pressure to Water Depth: Computes approximated depth of water where the pressure would occur.
- Bernoulli's Equation (Pressure): Uses Bernoulli's equation to compute fluid pressure (p1) based on the fluid velocities, heights and second pressure (p2).
- Bernoulli's Equation (Velocity): Uses Bernoulli's equation to compute fluid velocity (v1) based on the pressures, heights and second velocity (v2).
- Bernoulli's Equation (Elevation): Uses Bernoulli's equation to compute elevation (h1) based on the pressures, fluid velocitities and second height (h2)
- Volume of Water in a Well: Compute the volume of water in a well based on the static water level and the diameter of the well pipe..
- Pressure to Water Depth: Computes the height needed to acheive a desired pressure considering the density of liquid (e.g., water).
- Pressure Head: Computes the pressure based on the height of Gravity-Fed water from a tank or resevoir.
- Underwater Pressure: Computes the added pressure exerted underwater by the water column directly overhead as a function of the density of the water, depth under water, and the acceleration due to gravity.
- Heat Load and Water Flow: Computes the ton of heat capacity associated with a volumetric flow of water and a change in temperature.
References
Whitten, et al. "Chemistry" 10th Edition. Pp. 530
Wikipedia - Osmotic Pressure